350 Health Professionals Get Training on Medellin’s Low-Cost Covid-19 Respirator Technologies
The Medellin Mayor’s Office announced June 11 that 350 health professionals are now being trained on how to use new, relatively low-cost respirators developed by the ‘InnspiraMED” initiative for Covid-19 victims.
“This training takes place prior to the delivery of low-cost mechanical respirators developed by the InnspiraMED initiative, which is articulated by [Medellin technology incubator] Ruta N and financed by [bottled beverages giant] Postobón,” according to the Mayor’s Office.
“About 350 health professionals in Colombia including general practitioners, internists, anesthesiologists, emergency physicians and respiratory therapists participate in this course.
“The Faculty of Medicine of the University of Antioquia, in coordination with the Universidad Pontificia Bolivariana, the Universidad Cooperativa de Colombia and SENA through their simulation laboratories are part of this training process,” according to the Mayor’s press bulletin.
The training includes “correct use of personal protection elements, technical skills for advanced airway management and use of ventilators, as well as the development of clinical cases for problem-solving and decision-making in patient management,” according to the bulletin.
“Under a clinical simulation model, 150 health professionals from the Aburrá Valley [metro Medellin] are trained in-person, while another 200 professionals from 25 municipalities and capitals of the country such as Leticia (Amazonas), Tumaco (Nariño), Plato (Magdalena), Chiriguaná ( Cesar) and Lérida (Tolima), among others, will do so virtually.
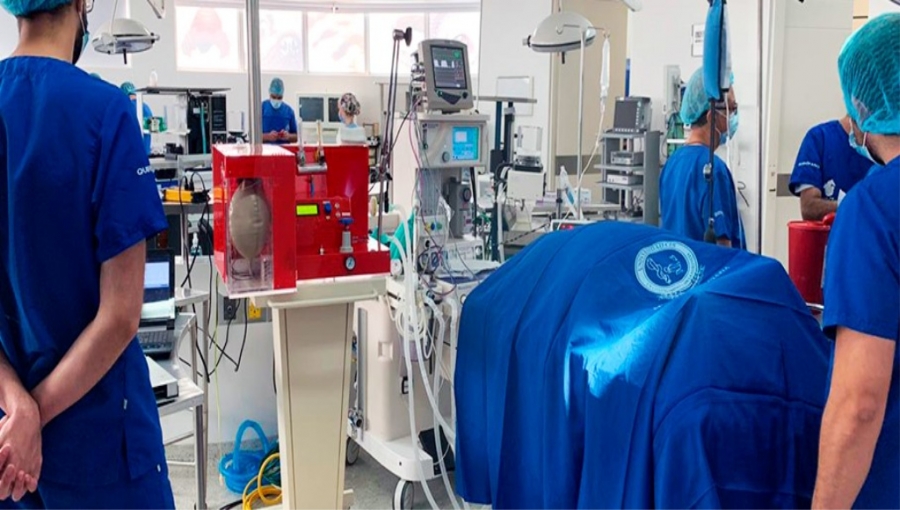
“By means of an anatomical model that behaves in the same way that a patient would under certain circumstances, the training will allow medical simulations to be carried out in which the assistants will delve into the management of patients with respiratory distress caused by Covid-19.”
“The production of low-cost mechanical ventilators is one of the main tools that the country has to face the crisis caused by Covid-19,” added Gabriel Sánchez, manager of the InnspiraMED initiative.
Invima in ‘Extraordinary Session’ for Ventilator Approvals
Meanwhile, Invima — Colombia’s national medical-device approval agency – announced June 11 that a special commission in charge of Covid-19 ventilator evaluations is now in “permanent extraordinary session until the initiatives of prototype respirators meet all requirements.”
“After evaluating development of novel Covid-19 respiratory devices from June 4 to June 9, 2020, the specialized commission on medical devices and in-vitro diagnostic reagents of the Review Committee declares itself in a permanent, virtual extraordinary session until the requirements on the research protocols of the ‘InnspiraMED’ and ‘Unisabana Herons’ projects are rectified,” according to Invima.
The decision is based upon the “importance of these medical devices in the current health emergency and the need to supply their evaluation with rigorous technical-scientific protocols,” according to Invima.
“The approval of research protocols of the projects by the specialized review commission is necessary to start the clinical research phase with human beings. Herein lies the importance of making this evaluation quickly, but with all the sanitary rigor, so as to mitigate any risk in their use.
“Thanks to the permanent monitoring by the specialized commission, it has been verified that the projects present significant progress, within the framework established by international norms for manufacturing these medical devices.”
However, the novel ventilators still must go through further evaluations — in part to ensure that Colombia’s “EPS” health insurance networks indeed will approve their use and then reimburse the clinics and hospitals that would employ such technologies, Invima added.